Requirements:
(a) Glassware: Nesslers’ cylinder, measuring cylinder and glass rod.
(b) Chemicals: Citric acid (20%), thioglycollic acid, ammonia solution (10%), standard iron solution and sulphuric acid.
Principle for Limit test for Iron
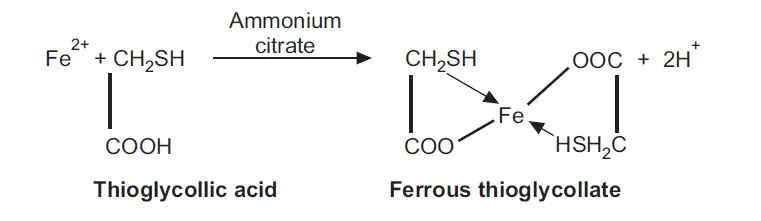
Limit test of Iron is based on the reaction of iron in ammonical solution with thioglycollic acid in presence of citric acid to form iron thioglycolate which is pale pink to deep reddish purple in color
Note:
1) Citric acid reacts with ammonia solution, giving ammonium citrate, which act as a buffer. The citrate ion also prevents the precipitation of iron by ammonia by forming a complex with it.
2) Thioglycollic acid not only reacts with ferrous ions, but it also reduces ferric ions to ferrous ions.
Reagent Preparations:
• Standard solution: Accurately weighed 0.1726 g of ferric ammonium sulphate is dissolved in 10 ml of 0.1N sulphuric acid (H2SO4) and the volume make up to 1000 ml with water.
• Citric acid (20%): Dissolve 20g of iron free citric acid in 100 ml of distilled water.
• Iron free ammonia solution: Dilute ammonia solution i.e. 10% ammonia
Procedure:
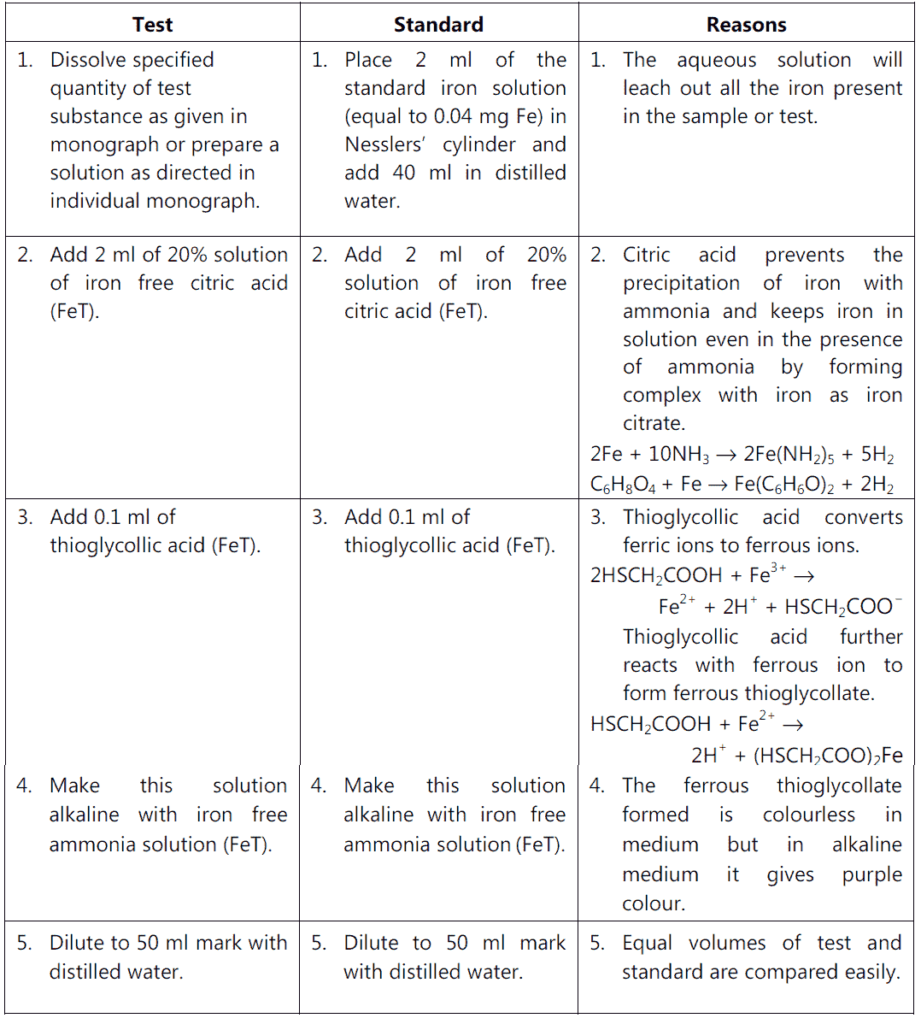
Conclusion:
If the intensity of purple colour in standard is more than that in the test, the sample complies limit test of iron as per I.P. 1996.
Also Read…. Limit test for Sulphate
Join Our WhatsApp Group to receive the latest updates like Pharma Job notifications, study materials, admission alerts, Pharma News, etc
Join Our Telegram Group to receive the latest updates like Pharma Job notifications, study materials, admission alerts, Pharma News, etc
Join Our Telegram Group to Download Free Books & Notes, Previous papers for D.Pharm, B.Pharm, M.Pharm, Drug Inspector & GPAT……….

Comments are closed