“Limit test is defined as a quantitative or semi-quantitative test designed to identify and control small quantities of impurity which is likely to be present in the substance. The limit test is generally carried out to determine the inorganic impurities present in the compound”.
In short, a limit test is nothing but to identify the impurities present in the substance and to compare it with the standard.
Importance of Limit test:
- To find out the harmful amount of impurities.
- To find out the permissible/impermissible amount of impurities.
Requirements of reasant for limit test of Chloride
(a) Glasswares: Nesslers’ cylinder, measuring cylinder and glass rod.
(b) Chemicals: Dil. Nitric acid (10%), 0.1 M silver chloride, distilled water.
The chloride limit test is designed to determine the allowable limit of chloride present in a sample.
Principle of limit test of Chloride
Limit test of chloride is based on the precipitation reaction. The precipitates of chlorides develop on a reaction of soluble chloride with silver nitrate in the presence of dilute nitric acid to form silver chloride, which appears as solid particles (opalescence) in the solution. The intensity of turbidity depends on the number/amount of chlorides present in the test substance/sample.
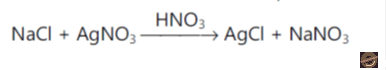
Reagent Preparations:
- Dil. Nitric Acid: 106 ml conc. HNO3 is diluted to 1000 ml with water.
- Silver Nitrate Solution: 5 g of AgNO3 is dissolved in 100 ml of water.
- Standard Sodium Chloride solution: Dissolve 0.05845 g of NaCl in 100 ml distilled water.
- Test sample:
- Dextrose: Dissolve 1 g in 10 ml distilled water to make the test sample.
- Sodium Bicarbonate: Dissolve 1 g in 10 ml distilled water.
Procedure for limit test of Chloride
| Specific weight of compound/ test sample is dissolved in water or solution is prepared as directed in the pharmacopeia and transferred in Nesslers’ cylinder. | Take 1 ml of 0.05845 %w/v solution of sodium chloride in Nesslers’ cylinder. | The aqueous solution will leach out all the chloride ions present in the sample and make them ready to react with silver nitrate. |
| Add 10 ml of dil. nitric acid. | Add 10 ml of dil. Nitric acid. | Dil. nitric acid is added in the limit test of chloride to make the solution acidic and helps silver chloride precipitate to make solution turbid at the end of process |
| Dilute to 50 ml in Nesslers’ cylinder | Dilute to 50 ml in Nesslers’ cylinder | For comparison of opalescence, an equal volume of both is taken. |
| Add 1 ml of 0.1 M AgNO3 solution, stir properly and keep aside for 5 min. | Add 1 ml of 0.1 M AgNO3 solution, stir properly and keep aside for 5 min. | The Ag+ ions will react with Cl- ions to form opalescence of silver chloride (as shown in priciple) |
| Observe the opalescence/turbidity. | Observe the opalescence/turbidity. |
Conclusion:
If opalescence produced in the sample solution is less than the standard solution, the sample will pass the limit test of chloride and vice versa.
Viva Question
Q.1: Why come turbidity in chloride limit test?
Limit test of chloride is based on the precipitation reaction. The precipitates of chlorides develop on reaction of soluble chloride with silver nitrate in the presence of dilute nitric acid to form silver chloride, which appears as solid particles/turbidity (opalescence) in the solution. The intensity of turbidity depends on the amount of chlorides present in the test substance.
Join Our WhatsApp Group to receive the latest updates like Pharma Job notifications, study materials, admission alerts, Pharma News, etc
Join Our Telegram Group to receive the latest updates like Pharma Job notifications, study materials, admission alerts, Pharma News, etc
Join Our Telegram Group to Download Free Books & Notes, Previous papers for D.Pharm, B.Pharm, M.Pharm, Drug Inspector & GPAT……….


Comments are closed