QUALITY CONTROL TESTS
Quality control of Aerosol tests include the following testing parameters of:-
1. Propellents
2. Valves, Actuator, Dip Tubes
3. Containers
4. Weight Checking
5. Leak Testing
6. Spray Pattern Testing
1. Propellent
Vapor pressure and density of the propellant are determined and compared with-specification sheet. Parameter Tested By Identification Gas Chromatography IR Spectroscopy Purity and acceptability Moisture, Halogen, Non-Volatile Residue determinations
2. Valves, Actuator, Dip Tubes
- Sampling is done according to standard procedures as found in Military Standards ―MIL-STD-105D
- For metered-dose aerosol valves, test methods were developed by
- Aerosol Specifications Committee‘
- Industrial Pharmaceutical Technology Section
- Academy Of Pharmaceutical Sciences
- The objective of this test is to determine magnitude of valve delivery & degree of uniformity between individual valves.
- Standard test solutions were proposed to rule out variation in valve delivery.
3. Containers
Containers of aerosol are made of tin-plate, aluminum, stainless steel, and certain types of glass. One of the important criteria for the selection of an aerosol container is withstood internal created pressure as high as 140–180 psi at 130°F.
Both the coated and uncoated containers are evaluated for defects in the inner lining. Quite a lot of quality control aspects and assessment parameters are applied according to the type of container used.
4. Weight Checking
This test is done to check the accuracy of the filling procedure and assurance of uniformity of the final weight of the product.
5. Leak Testing
Leal testing is done by determining the crimp’s measurements.
This test involves allowing the filled containers passing through a water bath and final testing of valve closure is done. Pass the crimped aerosol containers through the water bath. If any leaks are present, the evolution of air bubbles can be observed and the container is rejected.
Bestowing to this test, leakage is defined as the weight change of the same container before and after being stored in a vertical position at 25°C±2°C for a minimum of 3 days. According to the USP-NF, 12 pressurized containers selected at random, aerosol dispensers are selected and weighed in mg, and this is considered as the weight before the positioning (W1). Allow the filled tested container to stand in an upright correct position at room temperature for at least 3 days, and weigh of each container should be determined again, finding the weight in mg of each container as (W2). The time during which the containers were under test (T) is determined in hours. Ultimately, the leakage rate of each container is calculated in mg/year as follows.
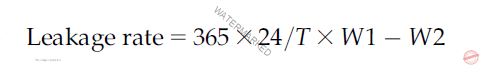
6. Spray Pattern Testing
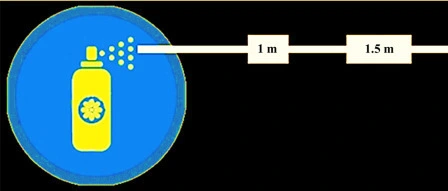
This test is carried out to inspect for defects in valves and spray pattern as well as to clear dip tube of pure propellant &concentrate .The sprayed particles that strike on the surface of the substrate testing paper cause the dye to be converted into solution and to be absorbed onto the paper.
Evaluation of Packaging components of Aerosol
I. Protection
II. Compatibility
III. Safety
I. Protection
- A light-resistant container that proposed to offer protection from light was evaluated by performing the Light transmission test.
- Water Vapor Permeation test, the capability of a container closure system to protect against transmission of moisture could be evaluated.
II. Compatibility
A container component must be compatible with a dosage form/medicament.
III. Safety
For this test, the containers is evaluated for physicochemical tests.
Also, Read……..
AEROSOLS EVALUATION PARAMETERS
Components of Pharmaceutical Aerosol
Join Our WhatsApp Group to receive the latest updates like Pharma Job notifications, study materials, admission alerts, Pharma News, etc
Join Our Telegram Group to receive the latest updates like Pharma Job notifications, study materials, admission alerts, Pharma News, etc
Join Our Telegram Group to Download Free Books & Notes, Previous papers for D.Pharm, B.Pharm, M.Pharm, Drug Inspector & GPAT……….

Comments are closed