karl Fischer titration is a analytical titration method used to determine the quantity of water/moisture content present in a solid, liquid & gaseous sample.
Karl Fisher Reagent
Karl Fischer reagent which is composed of iodine, sulphur dioxide, pyridine (a base) and methanol (as solvent). Both pyridine and methanol should be anhydrous.
Solvent: Alcohol (dehydrated methanol)
Buffering agent: Pyridine (a base)
Mix in a 750 ml combustion flask 400 ml of dehydrated methanol and 80 g of dehydrated pyridine. Immerse the flask in ice and bubble dried sulphur dioxide slowly through the mixture until its weight has increased by 20 g. Add 45 g of iodine and shake until it dissolves. Allow the solution to stand for 24 hours before use. This solution deteriorates gradually; therefore standardise it within 1 hour before use. Protect from light while in use.
Store any bulk stock of the reagent in a suitably sealed, glass stoppered container, fully protected from light and under refrigeration.
Stability of the Reagent: The stability of the original Karl Fischer reagent initially prepared with an excess of methanol was found to be fairly poor and hence, evidently needed frequent standardization. However, it was estabtished subsequently that the stability could be improved significantly by replacing the methanol by 2-methoxyethanol.
the following precautions must be observed rigidly using the Karl Fischer reagent, namely-
(a) Always prepare the reagent a day or two before it is to be used,
(b) Great care must be taken to prevent and check any possible contamination either of the reagent or the sample by atmospheric moisture,
(c) All glassware(s) must be thoroughly dried before use,
(d) Standard solution should be stored out of contact with air, and
(e) Essential to minimise contact between the atmosphere and the solution during the course of titration.
Principle of Karl Fisher Titration
Principle of KF titration involves oxidation of sulfur dioxide by Iodine in the presence of water, in a buffered solution.
Water present in the analyte reacts with the Karl Fischer reagent in a two-stage process as shown below :

Oxidation of sulphur dioxide takes place by iodine (Oxidation reaction between sulphur dioxide & iodine) to form sulphur trioxide and hydrogen iodide there by consuming one mole of water. this reaction is also known as Bunsen reaction.
Each one molecule of iodine disappears against each molecule of water present in the given sample. i.e. Water and iodine are consumed in a 1:1 ratio in the above reaction.
Once all of the water present is consumed, the presence of excess iodine is detected voltametrically by the titrator’s indicator electrode. That signals the end-point of the titration.
Type of of Karl Fischer Titration
Two Types of Karl Fischer Titration– Volumetric KFT & Coulometric KFT.
Volumetric KFT
– Iodine is added mechanically to a solvent containing the
sample
– Water is quantified from the volume of KF reagent
consumed
– 100 to 1×106 ppm (0.01 – 100%)
Coulometric KFT
– Iodine is generated electrochemically in situ during the
titration
– Water is quantified from the total charged passed (Q) as measured by current (amperes) and time (seconds), according to the following relationship:
Q = 1 C = 1 A x 1 S where 1 mg H2O = 10.72 C
– 1 to 50,000 ppm (0.0001 – 5%)
Karl Fischer titration apparatus
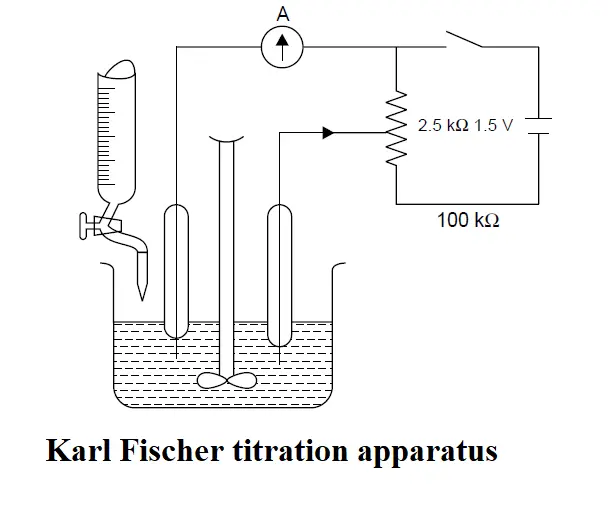
Karl Fischer titration apparatus is a a simple dead-stop end-point assembly.
The titration vessel is fitted with a pair of identical platinum electrodes, a mechanical stirrer with adjustable speed, and a burette. It will be observed that absolutely little or no current may flow unless and until the solution is totally free from any polarizing substances ; this could perhaps be due to the absorbed layers of oxygen and hydrogen on the anode and cathode respectively. However, the current shall flow only when the two electrodes get depolarized. The Karl Fischer reagent is pumped into the burette by means of hand bellows, the exccess of moisture is usually prevented by employing an appropriate arrangement of desiccant tubes. Alternatively, the stirring may also be accomplished either by using a magnetic stirrer or by means of a suitably dried nitrogen passed gently through the solution during the course of titration.
The end-point is achieved by employing an eiectrical circuit comprising of a microammeter (A), platinum electrodes, together with a 1.5 V to 2.0 V battery connected across a variable resistance of about 2.5 kΩ. First of all the resistance is adjusted in such a manner that an initial current passes through the platinum electrodes in series with a microammeter (A). After each addition of reagent, the pointer of the microammeter gets deflected but quickly returns to its original position. At the end of the reaction a deflection is obtained which persists for 10-15 seconds.
End-point Detection:-
The end-point of the Karl Fischer titration may be determined quite easily by adopting the electrometric technique employing the dead-stop end-point method. When a small quantum of e.m.f. is applied across two platinum electrodes immersed in the reaction mixture, a current shall tend to flow till free iodine exists, to remove hydrogen and ultimately depolarize the cathode. A situation will soon arise when practically all the traces of iodine have reacted completely thereby setting the current to almost zero or very close to zero or attain the end-point.
Limitations of Karl Fischer Titration:-
The Karl Fischer titration has a number of serious limitations due to possible interferences tantamount to erroneous results, namely-
(i) Oxidizing agents, for instance : chromates, Cu(II), Fe(III), Cr2O72–, peroxides, salts, higher oxides,
Example :
MnO2 + 4C5H5NH+ + 2I– → Mn2+ + 4C5H5N + I2 + H2O
(ii) Reducing agents, such as : Sn(II) salts, sulphides, and S2O32–
(iii) Compounds that have a tendency to form water with the ingredients of the Karl Fischer reagent, for instance :
(a) basic oxides : e.g., ZnO ;
Example : ZnO + 2C5H5NH+ → Zn2+ + C5H5N + H2O
(b) salts of weak oxy-acids e.g., NaHCO3 ;
Example : NaHCO3 + C5H5NH+ → Na+ + H2O + CO2 + C5H5N
Note : H2CO3, carbonic acid, is very unstable ; hence it splits up to yield a mole each of water and CO2.

Comments are closed