Antidotes are drugs designed to counteract toxins/poison/xenobiotics.
An antidote can act in a number of ways like
- Limiting absorption
- Sequestering the poison
- Inhibiting metabolism to a toxic metabolite
- Promoting distribution from tissues
- Displacing the poison from a receptor or competing for the receptor
- Counteracting the toxic effect
- Enhancing detoxification
Classification of Antidote
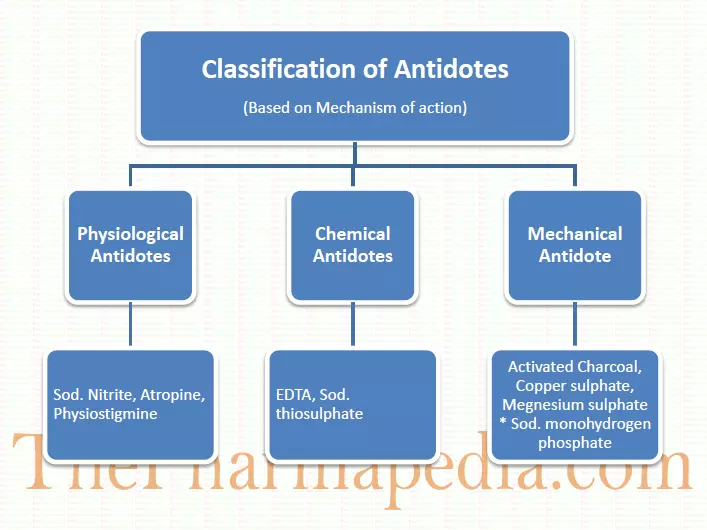
According to mechanism of action antidote are classified below
Physiological antidotes
Physiological antidote also called antagonist. The produce the effect opposite to that of the poison. They are used after some of poison is observed in the circulation.
Example–
Sodium nitrite (used in cyanide poisoning), atropine and Physiostigmine are two antidotes for each other.
Chemical antidotes
These antidotes react by combining with the poison and change its chemical nature by converting the poison into inactive or harmless compounds.
Examples
Sodium thiosulfate (it convert the systemic toxic cyanide into non toxic thiocyanate), EDTA (chemical agent for heavy metal poisoning).
Mechanical antidotes
They act by preventing the absorption of poison into the body or expel out the poisoning by emesis or eliminate through urine.
Example–
Activated charcoal absorb the poison prior to absorption into the systemic circulation form intestinal wall.
Copper sulphate, magnesium sulphate and sodium monohydrogen phosphate in activate the poison prior to absorption and precipitate the toxic material as in insoluble salt
Antidote for selected inorganic poison and drug overdose
| Poison/Drug Overdose | Antidote | Mechanism |
|---|---|---|
| Acid (Corrosive) | Antacid or Weak Alkali (Milk of magnesia), Avoid Induce vomiting | Chemical Antagonism, Acid base neutralization |
| Alkali (Caustics) | Weak Acid (Lemon juice/diluted vinegar) | Chemical Antagonism, Acid base neutralization |
| Iron Salts | Desferroxamine | Chelation |
| Copper & Leads | Penicillamine | Chelation |
| Mercury Salt | Dimercaprol (BAL) | Chelation |
Sodium Nitrite
M.F. : NaNO2; Mw; 69
Preparation
Most common suitable method
Absorbing of nitrogen oxide gas (NO) by sodium carbonate solution. The solution is concentrated to crystallize out the product.
2Na2CO3 + 4NO + O2 → 4NaNO2 + 2CO2
Properties
- Colourless to slightly yellow crystals, odourless, saline taste.
- It is deliquescent. Absorb moisture and slowly gets oxidized to sodium nitrate.
- Soluble in water.
- It is a reducing agent.
Use
- As reducing agent when combine with Sodium carbonate to form anti-Rust tablets. Anti-Rust tablets are used to prevent rusting of surgical instruments.
- As antidote in the treatment of cyanide poisonings
Cyanide poisoning & its treatments
Join Our WhatsApp Group to receive the latest updates like Pharma Job notifications, study materials, admission alerts, Pharma News, etc
Join Our Telegram Group to receive the latest updates like Pharma Job notifications, study materials, admission alerts, Pharma News, etc
Join Our Telegram Group to Download Free Books & Notes, Previous papers for D.Pharm, B.Pharm, M.Pharm, Drug Inspector & GPAT……….
