Disinfectants may lose potency on standing and addition of organic matter, so that their efficacy must be tested.
Disinfectants must be tested periodically to ascertain their potency and efficacy. Evaluation is not for concentration, But it is for the activity/efficacy of the agent under certain conditions like the presence of organic matters and uses.
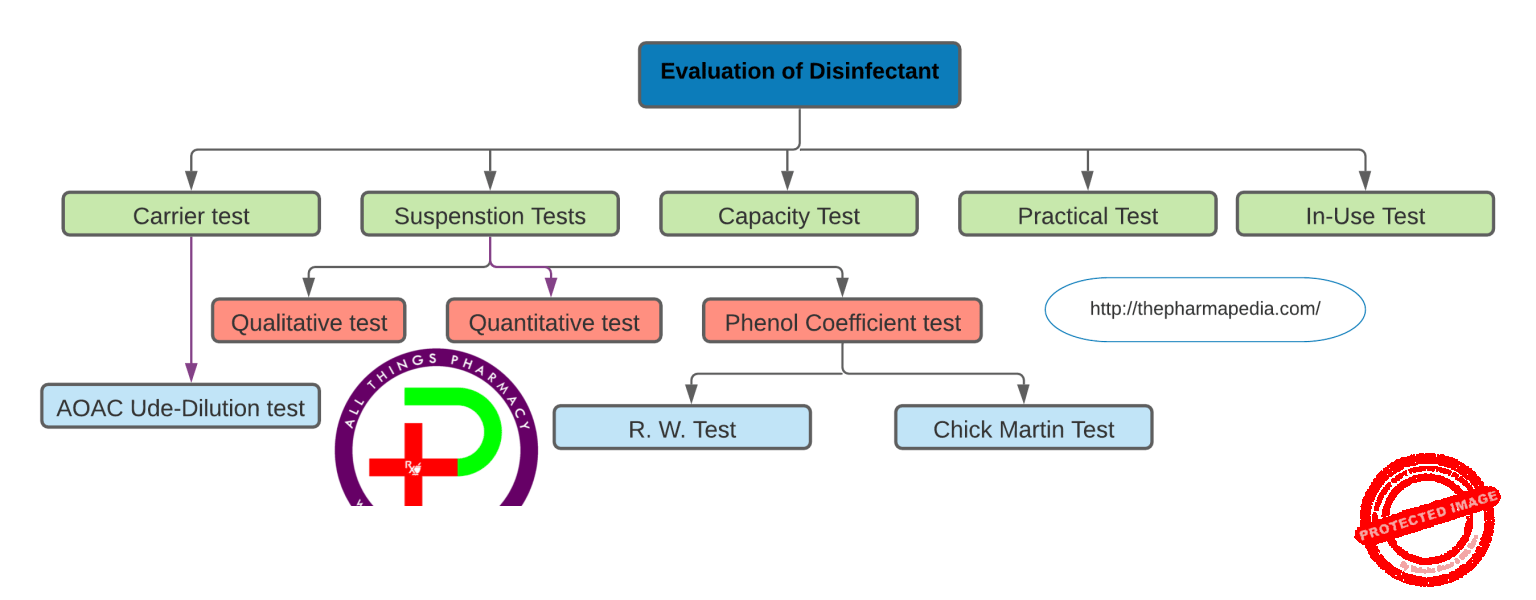
The standard tests to check disinfection efficiency include:-
- Suspension tests (Rideal-Walker phenol coefficient/R.W.C) test )
- Chick-Martin and Garrod’s test
- This test also determines the phenol coefficient of the test disinfectant. Unlike in the Rideal Walker method where the test is carried out in the water, the disinfectants are made to act in the presence of yeast suspension (or 3% dried human feces) to simulate the presence or organic matter.
- Kesley and Maurer’s in-use tests
- Capacity Use Dilution test (carrier-based test)by Kelsey and Sykes/Capacity test of Kelsey-Sykes -triple challenge test and
- Other microbial time-kill assays (Disinfectant kill time test)
Evaluation methods of Disinfectants
Factors affecting the disinfection process:
- Effect of temperature:
- Bacteriologist Koch had noted that anthrax spore were more readily killed by the same concentrations of phenol if the temperature was elevated.
- Effect of pH: Any change in PH will affect:
- Rate of growth of bacteria >> growth is optimal at pH (6-8).
- Potency of antimicrobial agent >> due to ionization at pH.
- Ability of drugs to combine with cell surface sites of bacteria.
- Effect of surface activity:
- The addition of low concentrations of surface-active compounds may potentiate the biological effect of an antibacterial agent.
- Presence of interfering substances;
- The presence of other material may reduce the effect of such an agent by adsorbing or inactivating it and thus reducing the amount available for combining with the cells it is desired to kill.
Evaluation methods of Disinfectants
1. Carrier test
Principle:
• A carrier such as a silk or catgut thread is contaminated by submersion in a liquid culture of the test organism.
• The carrier is then dried and brought in contact with the disinfectant for a given exposure time.
• cultured in a nutrient broth;
• No growth indicates the activity of the disinfectant tested whereas growth indicates a failing.
• By multiplying the test concentrations of the disinfectant and the contact times, a potentially active concentration-time relationship of the disinfectant is obtained.
Limitations
a) The number of bacteria dried on a carrier is hard to standardize
b) The survival of the bacteria on the carrier during drying is not constant.
The AOAC Use-dilution test
• AOAC (American Association of Official Analytical Chemists)
• A carrier-based test.
• Organisms: Salmonella cholerasuis, S. aureus and P. aeruginosa.
• Carriers (stainless steel cylinders) are meticulously cleaned, sterilized, cooled and inoculated with a test organism by immersing in one of the culture suspensions.
• The cylinders are drained on filter paper, dried at 37°C for 40 minutes, exposed to the use-dilution of the disinfectant for 10 minutes.
• After transfer from the disinfectant, the treated test surfaces are incubated in the neutralizing growth medium for 48 hours.
• The number of tubes showing growth of the target microorganism is recorded.
• To “pass” a 60 carrier test, at least 59 of the 60 surfaces tested must demonstrate complete disinfection (no detectable growth of the target microorganism in the test tubes containing neutralizing growth medium).
• To “pass” a 10 carrier test, complete disinfection must take place on all test surfaces.
2. Suspension tests
Principle:
• A sample of the bacterial culture is suspended into the disinfectant solution
• After exposure it is verified by subculture whether this inoculum is killed or not.
• Suspension tests are preferred to carrier tests as the bacteria are uniformly exposed to the disinfectant.
Types of Suspension Test:
a) Phenol Coefficient Test (Determined by two methods-Rideal Walker/R.W. method & Chick Martin test)
b) Qualitative test
c) Quantitative test
a) Phenol Coefficient Test
The phenol coefficient of a disinfectant is calculated by dividing the dilution of the test disinfectant by the dilution of phenol that disinfects under predetermined conditions.
Phenol Coefficient is determined by two methods:
b) Qualitative test
• A loopful of bacterial suspension brought into contact with the disinfectant
• A loopful of this mixture cultured for surviving organisms.
• Results expressed as ‘growth’ or ‘no growth’.
c) Quantitative test
• The number of surviving organisms (B) is counted and compared to the original inoculum size (A).
Microbicidal effect (ME) = Log (A) – Log (B)
ME = 1 → killing of 90% of the initial number
ME = 2 → 99% killed.
• A generally accepted requirement is ME ≥ 5 →99.999% of the germs are killed.
3. Capacity tests/Kelsey-Sykes test
• The ability to retain activity in the presence of an increasing load is the capacity of the disinfectant.
• In a capacity test, the disinfectant is challenged repeatedly by successive additions of bacterial suspension until its capacity to kill has been exhausted.
• Capacity tests simulate the practical situations of housekeeping and instrument disinfection.
• Best known capacity test is the Kelsey-Sykes test
Kelsey-Sykes test
• A triple challenge test, designed to determine concentrations of disinfectant that will be effective in clean and dirty conditions.
• Organisms: 4 organisms (S. aureus, E.coli, P. aeruginosa and Proteus vulgaris)
• Three successive loads of bacteria (additions) (0, 10, and 20 min)
• Temp: 20oC
4. Practical tests
• The practical tests under real-life conditions are performed after measuring the time concentration relationship of the disinfectant in a quantitative suspension test.
• The objective is to verify whether the proposed use dilution is still adequate in the conditions under which it would be used.
• The best-known practical tests are the surface disinfection tests.

