Sterilization can be defined as any process that effectively kills or eliminates transmissible agents (such as fungi, bacteria, viruses and prions) from a surface, equipment, foods, medications, or biological culture medium. Sterility can be achieved by exposure of the object to be sterilized to chemical or physical agent for a specified time. Moist heat sterilization is the most efficient biocidal agent.
or
In simple words, Sterilization is an inactivation of Micro-organism (MOs) by physical or chemical means.
Pharmaceutical Importance of Sterilization
Terms commonly used in Sterilization Process
D-value
The D value is a single quantitative expression of the rate of killing of microorganisms.
For radiation and heat treatment, D-value is the time taken at a fixed temperature or the radiation dose required to achieve a 90% reduction in viable count. or This is the time required for a 90% reduction in the microbial population. Hence, the time or does it takes to reduce 1000 microbial cells to 100 cells is the D value.
D-value is indicative of the resistance of any organism to a sterilizing agent.
D value is important in the validation of sterilization process for several reasons.
- It is specific for each microorganism in environment subjected to specific sterilizing agent or condition.
- The knowledge of D value at different temperature in heat sterilization is necessary for the calculation of Z value.
- The D value is used in the calculation of biological factor F.
- Extra-polation of D value predicts number of log reduction of microbial population.
D value is affected by several parameters which are as follows.
- The type of microorganism used as biological indicator.
- The formulation component and characteristics
- The surface on which the microorganism is exposed
- The temperature, gas concentration and radiation dose
Z-value
Z-value represents the increase in temperature needed to reduce the D-value of an organism by 90%.
Sterilization Assurance limit (SAL): The product will be sterilized by a sterilization process sufficient to produce a probability of non-sterility of one out of 1 million containers i.e 10-6.
SAL= 10-6(1 viable microorganism in 106 sterilized items of the final product).
Whenever possible terminal sterilization is chosen, sterilized in its final container & possible with heat-stable particles.
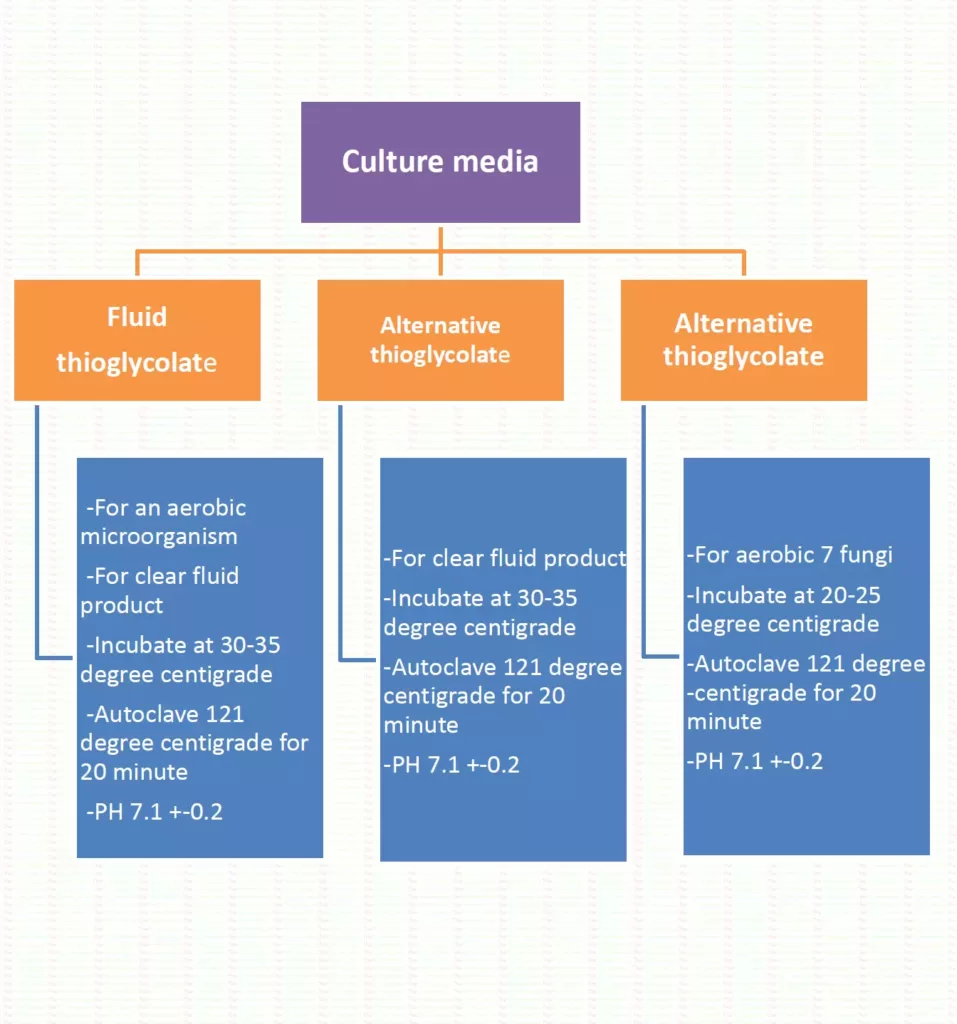
Sterility test
- Sterility test is applied to the Pharmaceutical preparations that are required to sterile preparation like parenteral and ophthalmic preparation.
- To detect the presence of viable forms of microorganisms.
- Sterility test is carried out at aseptic condition (grade A laminar airflow cabinet)
- The microorganism are placed in a culture medium that provides nutrition, water, and favorable temperature/condition, microorganism will grow and their presence is detected in the culture medium.
Read more about sterility test in details…
Method of sterilization

A. Physical Method
(a) Thermal (Heat) methods
i. Steam sterilization/moist heat sterilization/autoclave
- Used for aqueous & surgical materials.
- most widely used and reliable method of sterilization
- The efficiency to inactivate microorganisms is dependent upon the degree of heat, the exposure time and the presence of water.
- Sterilization is carried out using saturated steam under pressure. Saturated steam cause destruction of enzyme and other cellular component and protein.
- Autoclaving also kills the virus
- not suitable for rubber, plastics, and equipment that would be damaged by high temperatures
| Holding temp. | Holding time (minutes) | Pressure |
| 115-118 ℃ | 30 minutes | |
| 121- 124 ℃ | 15 minutes | |
| 126- 129 ℃ | 10 minutes | |
| 134-138 ℃ | 3 minutes |
ii: Dry heat sterilization/ Hot air Oven
- Used for sterilizing glass-wares, metal surgical instruments & sterilizing non-aqueous thermo-stable liquids and thermostable powders.
- Dry heat destroy bacterial endotoxin
- Pyrogen can destroy at 600 degrees centigrade for 60 second
| Holding temp. | Holding time |
| 160 ℃ | 2 hrs |
| 170 ℃ | 1 hrs |
| 180 ℃ | 30 minutes |
(b) Radiation method (Gamma radiation)
- Gamma radiation Cause ionization of DNA
- Dose: 2.5 Mrads
- Source of radiation: Cobalt 60 and cesium 137
- Many types of radiation are used for sterilization like electromagnetic radiation (e.g. gamma rays and UV-260 nm light), particulate radiation (e.g. accelerated electrons).The major target for this radiation is microbial DNA. Gamma rays and electrons cause ionization and free radical production while UV light causes excitation.
- Radiation sterilization is generally applied to articles in the dry state; including surgical instruments, sutures, prostheses, unit dose ointments, plastic syringes and dry pharmaceutical products.
- UV light, with its much lower energy, and poor penetrability finds uses in the sterilization of air, for surface sterilization of aseptic work areas, for treatment of manufacturing grade water, but is not suitable for sterilization of pharmaceutical dosage forms
(c) Filtration method
- Used for thermolabile/heat sensitive Drugs
- The size of filter medium pores to retain micro-organisms must be quite small. The 0.20- or 0.22 micrometer pore size filter media are considered to be capable of producing sterile filtrates.
- Both clarification and sterilization of liquid preparation
- Filtration process does not destroy but removes the microorganisms by adsorption and trapping within the matrix of the filter material.
- The bubble point test is a popular single-point physical integrity test for disc filter membrane. A filter medium is wetted with a liquid, and test gas pressure is slowly raised until a steady stream of bubbles appears from a tube or hose attached to the downstream side of the filter and immersed in water . The pressure at which the bubbles first appear is recorded as the bubble point and is related to the largest pores in the filter medium.
HEPA (High-efficiency particulate air)- filters can remove up to 99.97% of particles >0.3 micrometers in diameter. Application of filtration for sterilization of gases.
B. Chemical Method
(a) liquid
Hydrogen Peroxide Sterilization -oxidizing key cellular components, which inactivates the microorganisms.
(b) Gases
Ethylene oxide /(CH2)2O):
- Ethylene oxide is a colorless, odorless, and flammable gas.
- Composition: 10% Eth.Oxide+ 90% CO2
- Cause alkylations of sulphydryl, amino, hydroxyl and carboxyl groups on proteins and amino groups of nucleic acids/DNA.
- produce acute toxicity including irritation of the skin, conjunctiva and nasal mucosa.
- Efficacy depends upon humidity, time of exposure, temperature of exposure, concentration of gas, nature of microorganism.
- Limitation: Limited ability of Ethylene oxide gas to diffuse to the innermost product (penetrability problem)
Mechanism of various sterilization methods
| S. No. | Methods of Sterilization | Mechanism of action | Material to be sterilized |
|---|---|---|---|
| 1 | Moist Heat (steam) | Saturated steam under pressure-Destruction of enzyme, cellular component & Protein | Aq. & Surgical items, gloves, Aprons, Surgical dressing, Culture media |
| 2 | Dry heat | Oven or tunnel- Oxidative damage & denaturation of protein | Non-aq. & powders, metal surgical |
| 3 | Gas | Ethylene oxide-Alkylation of essential molecules | heat-sensitive |
| Vapor phase hydrogen peroxide- Oxidizing the cellular content | |||
| 4 | Radiation (cobalt-60, cesium-137) | Gamma rays and electrons cause ionization of DNA and free radical production while UV light causes excitation. | Sterilization of heat sensitive products |
| 5 | Filtration | Adsorption | Thermolabile |
Bio-logical indicators for Evaluation of Sterilization Methods
Biological indicators (BIs) are live spore forms of micro-organisms known to be the most resistant living organisms to the lethal effects of the particular sterilization process.
Provide the effectiveness of a given sterilization process/validation of the sterilization process.
Biological indicators are a viable cultures of known species of microorganisms.
| S. No. | Methods of sterilization | Biological indicator(Spores) |
| 1 | Moist Heat (steam) | Bacillus strearothermophilus |
| 2 | Dry heat | B. subtilis |
| 3 | Gas | B. subtilus |
| Bacillus stearothermophilus | ||
| 4 | Radiation (cobalt-60, cesium-137) | B. pumilus |
| 5 | Filtration | Pseudomonas dimunuta |
Also Read…

