Dental products
Dental products are those substances which prevent the dental caries, dental decay and give the freshness and cleanness to the mouth and teeth. In market it is mainly available in the form of toothpaste, tooth powder, mouthwash, tooth gel, dentifrice etc.
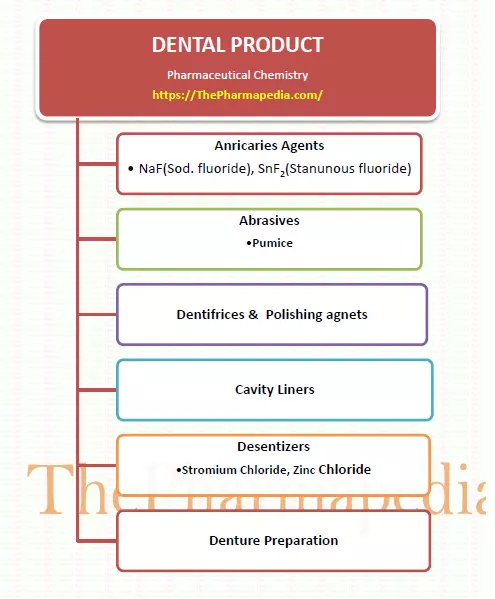
Dental Product Includes:-
- Anticaries Agents (NaF)
- Abrasives
- Dentifrices/Cleaning agents
- Cavity Liners
- Polishing agents
- Desensitizing agents
- Denture Preparation
Classification of Dental Product
On the basis of their activity it is divided into five parts
- 1. Antiplaque agent—
- Example: – Triclosan, delmiopinol, phenolic
compounds
- Example: – Triclosan, delmiopinol, phenolic
- 2. Anticaries agent—
- Example: – Sodium fluoride, stannous fluoride.
- 3. Cleaning/dentifrice agent —
- Example: – Calcium carbonate, calcium phosphate, sodium metaphosphate.
- 4. Desensitizing agent—
- Example: – Strontium chloride, zinc chloride.
- 5. Mouth washes—
- Example:- Chlorhexidine gluconate, potassium nitrate.
1. Anticaries agents
Agents used to prevent tooth decay.
Dental caries or tooth decay is caused by acids produced by the action of microorganism on carbohydrates.
It is believed that food particles containing carbohydrates lodged between the teeth undergo decay because of bacterial action and produce acids.
This disease is characterised by decalcification of tooth (calcium deposition) accompanied by foul mouth odour.
To prevent dental caries and to maintain clean and healthy teeth, it becomes necessary to use dentifrices, which clean the surface of teeth.
Example of Anticaries agents: – Sodium fluoride, stannous fluoride.

i) Sodium Fluoride (NaF)
Sodium fluoride is an inorganic chemical which is widely used for fluoride ion in dental products preparations. It protects the teeth from acid demineralization during bacterial growth. It provides the strength for tooth enamel and prevents the tooth decay. Minor quantity of sodium fluoride is used in drinking water.

Mol. Wt. 41.99
It contains not less than 98.5 percent and not more than 100.5 percent of NaF, calculated with reference to the dried substance.
Preparation
It may be prepared by neutralization of hydrofluoric acid with (HF) sodium carbonate ( Na2CO3 ).
2HF + Na2CO3 → 2NaF + H2O + CO2 (gas)
Another Method
It is prepared by double decomposition of calcium fluoride with sodium carbonate.
CaF2 + Na2CO3 → 2NaF + CaCO3
Properties
- It is a white powder.
- Colorless and odourless.
- It is soluble in water.
- Insoluble in alcohol.
- Upon acidification ; hydrofluoric acid is produced. :-NaF + HCl → HF + NaCl
Brand/Market Name—Optifresh, NuNof, D Flour, Vinaflour.
Assay
It is assayed by complexometric titration.
Weighed quantity is dissolved in water, sodium chloride and alcohol is added. Then the solution is treated with excess of lead nitrate.
2 NaF + pb (NO3)2 → pbF2 (ppt) + 2NaNO3
The precipitate is filtered. The filtrate and the washings are titrated with disodium edetate using xylerol orange as indicator.
Use
- To prevent dental caries.
- Usual dose:2.2 mg (equivalent to 1 mg of fluoride ion)
- Application: 1.5-3.0 ppm (equivalent to 0.7-1.3 ppm of fluoride ion) in drinking water; topically, as 2% solution to the teeth.
- Formulations: Sodium fluoride is administrated as solution, tablet, oral gel for systemic use or as mouth wash for local use.
Storage
- Should be stored in well closed container.
ii) Stannous fluoride
Molecular formula: SnF2 Molecular weight: 156.7
Tin fluoride solution is obtained from using tin fluoride capsules by dissolving in water. A fresh solution (about 8.0 %) finds use in dentistry.
Properties:
- It is a white crystalline powder having unpleasant astringent-salty taste.
- It is soluble in water but insoluble in alcohol and organic solvents.
- Aqueous solution of stannous fluoride deteriorates rapidly on standing because of oxidation of stannous cation to stannic form causing turbidity.
- Thus stannous fluoride solution must be freshly made.
Identification:
- To 5 ml of solution (1 in 100) in a test tube add 2 ml of calcium chloride test solution: a fine, white precipitate of calcium fluoride is formed.
- Mix on a spot plate 2 drops of solution (1 in 100) with 2 drops of silver nitrate test solution: a brown-black precipitate is formed.
Uses:
- It is used to prevent dental caries, similar to sodium fluoride and sodium monofluorophosphate in the form of solution, gel, mouth wash or dentifrice (toothpaste). It has unpleasant taste and may cause discolouration of teeth on overusage.
- A freshly prepared 8 % solution of stannous fluoride is applied to the cleaned teeth. A single application has been reported to be sufficient for six to 12 months.
2. Cleaning agents/Dentifrices
A dentifrice is a substance used with a tooth brush for the purpose of cleaning the surface of teeth.
Dentifrices contain agents for cleaning tooth surfaces and providing polishing effect on the cleaned teeth. These agents are abrasive in nature. They are responsible for physically removing plaque and debris. The overall effect provides whiteness to the teeth. Dentifrices are applied as powders or pastes.
- Examples:-
- Dicalcium phosphate,
- sodium metaphosphate,
- calcium pyrophosphate,
- calcium carbonate and
- calcium monohydrogen phosphate
Calcium carbonate (CaCO3)
It is the most abundant and widely distributed calcium salts. It occurs as chalk, marble, calcite, corals, pearls. aragonite, calcite, and limestone, marble.
Preparation:
It is prepared by precipitation by mixing the boiling solutions of calcium chloride and sodium carbonate and allowing the precipitation to cool.
CaCl2+ Na2CO3 → 2NaCl+CaCO3 ↓
or
Calcium carbonate is prepared by the reaction of calcium oxide with water and carbon dioxide. Initially water is added to calcium oxide then it forms calcium hydroxide. the carbon dioxide is passed through this solution to precipitate the desired calcium carbonate.
CaO (calcium oxide) + H2O → Ca(OH)2 (calcium hydroxide)
Ca(OH)2 + CO2 → CaCO3↓ + H2O
Properties—
➢ Color and state—it is colorless crystalline powder.
➢ Odor and taste—it is odorless with salty taste.
➢ Solubility—it is readily soluble in water but insoluble in alcohol.
Pharmaceutical preparation—Tablet, solution, drops, toothpaste.
Brand/Market Name—Optifresh, NuNof, D Flour, Vinaflour.
Uses
- Precipitated chalk, which is having a fine powdery texture, is used as dentifrice, both powders and pastes.
- It furnishes both abrasive effect in the mouth.
- Non-systemic antacid.
Storage condition— It is stored in well closed air resistance unopened
3. Desensitizing agents
- Desensitizing agents are used in dental preparations to reduce sensitivity of teeth to heat and cold.
- They act as local anesthetic.
- Example-
- Strontium chloride,
- Zinc chloride
Strontium chloride (SrCl2)
Preparation:
It is prepared by adding strontium carbonate to hydrochloric acid until effervescence gets ceased. The solution is filtered, concentrated and allowed to crystallise.
SrCO3 + 2HCl → SrCl2 + H2O + CO2
Uses: It acts desensitising agent in dental remedies.
4. Polishing agents
- Polishing is achieved by abrasive action (polishing and cleaning the hard surface) of dentifrices. It provides whiteness to the teeth.
- Example- sodium metaphosphat
5. Dental Cements and fillers
- Dental cements are used to temporarily cover protect areas that have undergo operation as in dental surgery.
- The cementing material is applied as a paste which gets hardened in a short while it can be removed by the dentist.
- The temporary cement is also medicated usually with eugenol which is antiseptic and local anaesthetic.
- Examle- Zinc oxide eugenol
Zinc oxide eugenol
- It is considered as the best cementing material in dental practice.
- When zinc oxide is made to mix with a strong solution of zinc chloride, it yields a mass which hardens within a short time which forms zinc oxychloride. A similar product can also be prepared by mixing zinc oxide with phosphoric acid resulting zinc oxyphosphate cement.
- Medicated cements can be prepared by adding suitable amounts eugenol or clove oil. This is used for temporary filling
Dental Caries (Tooth decay)
It is a disease of the teeth caused by acids produced by the action of micro organisms on food materials. This is disease is characterized by decalcification of tooth accompanied by foul mouth odour.
Dental caries, or tooth decay, involves a gradual demineralization (softening) of the enamel and dentin. If it is not treated then microorganisms may invade the pulp, causing inflammation and infection, with subsequent death of the pulp and abscess of the alveolar bone surrounding the root’s apex, requiring root canal therapy.
Dental caries (i.e. cavities) are formed by the growth and implantation of cariogenic microorganisms. Bacteria (primarily streptococcus mutans and lactobacillaceae) produce acids, mostly lactic acid that demineralize the enamel. The demineralized enamel initially appears as a white, chalky area and eventually becomes brown or yellow.
Diet is another factor in the development of dental caries. Diet with a high concentration of fermentable carbohydrates increases the risk of dental caries. Masses of bacterial cells, sticky polysaccharide (produced from sucrose) and other debris adhering to teeth constitute dental plaque. Fermentable sugar such as sucrose is converted by bacterial plaque into volatile acids that destroy the hydroxyapatite.
The formation of bacterial plaque also helps the decay process by forming pockets or crevices on the tooth surface in which the food particles can stick and be decayed by the bacteria. If plaque is not removed it calcifies into calculus when calcium salt precipitates from the saliva.
Brushing the teeth helps in removing the material from the tooth surface before it hardens into calculus. Dental caries can be prevented and oral and dental hygiene can be maintained with the help of dentifrices. These are the products that enhance the removal of stain and dental plaque by the toothbrush. The most accepted approach to prevent caries includes flossing and brushing accompanied by administration of fluoride either internally or topically to the teeth.
A number of inorganic compounds are used in maintaining the oral and dental hygiene. Most of them are over the counter (OTC) products. Dental products include anticaries agents (dentifrices and fluoride salts), polishing agents, and desensitizing agents.
To prevent dental caries and to maintain clean and healthy teeth it becomes necessary to use dentifrices. Main function of dentrifices is to clean the surface of the teeth.
Role of Fluoride
Small quantity of fluoride is necessary to prevent dental caries.. Fluoride is anticariogenic as it replaces the hydroxyl ion in hydroxyapatite with the fluoride ion to form fluorapatite in the outer surface of the enamel. Fluorapatite hardens the enamel and makes it more acid resistant.
Fluoride is most beneficial upto an age of 12 or 13 because unerupted permanent teeth are mineralizing during that time.
Fluoride can be administered by two routes, orally and topically. The fluoridation of the public water supply is the most convenient and effective may of oral administration. This can be achieved by adding sodium fluoride, giving a fluoride concentration of 0.7-1.0 ppm. Fluoride can also be administered orally as sodium fluoride tablets or drops added in water or fruit juice.
fluoride can be administered topically. A 2% aqueous solution of sodium fluoride is widely used topically. A freshly prepared 8% solution of stannous fluoride is also extensively used for topical application of fluoride.
Join Our WhatsApp Group to receive the latest updates like Pharma Job notifications, study materials, admission alerts, Pharma News, etc
Join Our Telegram Group to receive the latest updates like Pharma Job notifications, study materials, admission alerts, Pharma News, etc
Join Our Telegram Group to Download Free Books & Notes, Previous papers for D.Pharm, B.Pharm, M.Pharm, Drug Inspector & GPAT……….

Comments are closed