Types of Pharmaceutical Emulsion
Pharmaceutical emulsions are biphasic liquid preparations in which one liquid is dispersed as small globules in another immiscible liquid. They are stabilized by emulsifying agents to maintain uniform dispersion and physical stability.
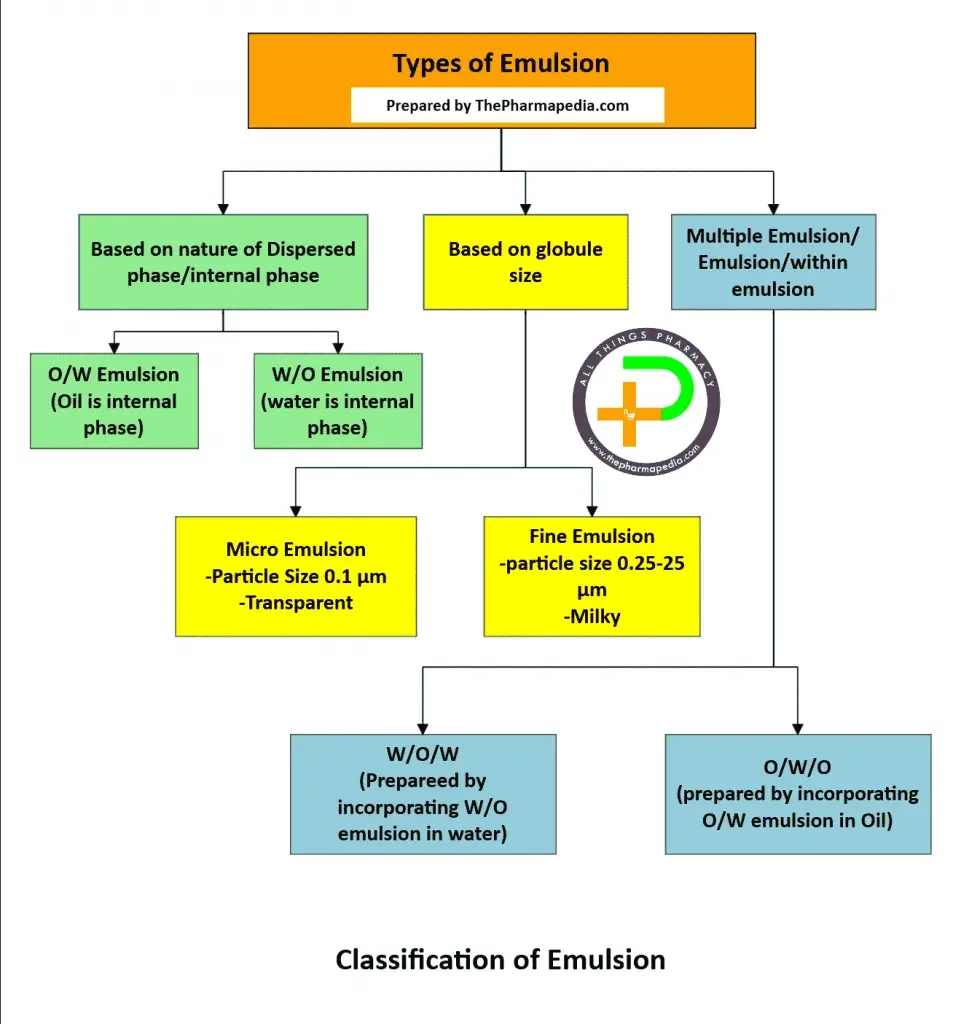
The classification of pharmaceutical emulsions is mainly based on the nature of internal and external phases, globule size, and complex structure.
Emulsions typically consist of a polar (aqueous) and a relatively nonpolar (an oil) liquid phase.
A. 🧪Based on Nature of the Internal & External Phase
Based on nature of the internal & external phase, emulsion can be classified into two types.
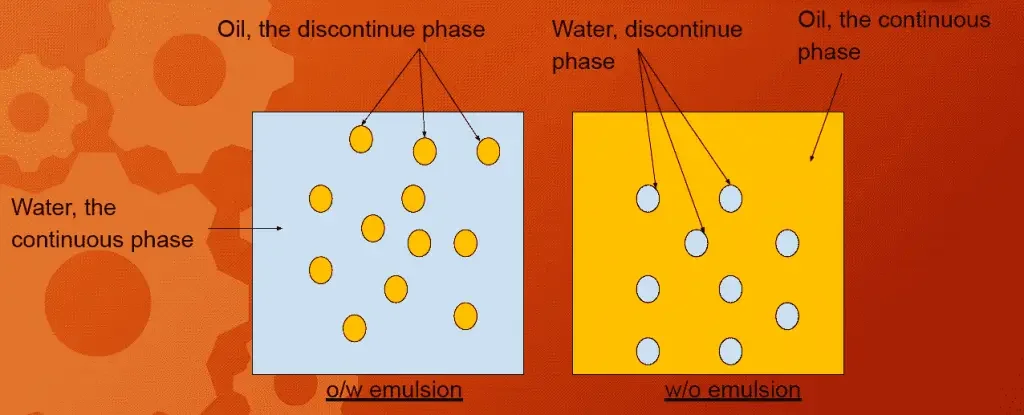
Emulsions can be classified into two main types depending on which phase acts as the continuous or dispersed medium.
1. Oil-in-Water (O/W) Emulsion
In this type, the oil phase is dispersed as globules throughout an aqueous continuous phase.
The emulsifier is present in the water (external phase) and helps stabilize the oil globules at the interface.
Examples of Hydrophilic Emulsifiers:
Sodium lauryl sulfate, triethanolamine stearate, sodium oleate, glyceryl monostearate.
Applications:
Used for internal use such as oral, topical, and parenteral formulations where water is the external phase.
2. Water-in-Oil (W/O) Emulsion
In this type, the aqueous phase is dispersed, and the oil phase acts as the continuous phase.
A lipophilic emulsifier is used to stabilize this system.
Examples of Lipophilic Emulsifiers:
Calcium palmitate, sorbitan esters (Spans), cholesterol, wool fats.
Applications:
Mainly used for external applications such as creams and ointments due to their oily nature and protective film formation.
B. ⚪Based on Globule Size
Depending on the particle or globule size of the dispersed phase, emulsions are classified as:
1. Micro Emulsion
- Globule size: about 0.01 µm
- Transparent in appearance due to the very small droplet size that does not refract light.
- Microemulsions are thermodynamically stable systems with excellent drug solubilization properties.
2. Fine Emulsion
- Globule size: 0.25 to 25 µm
- Milky in appearance due to larger droplet size.
Fine emulsions are commonly used in oral and topical formulations.
C. Multiple Emulsions
Multiple emulsions are complex systems in which the dispersed phase contains droplets of another emulsion — essentially “emulsions within emulsions.”
They are of two types:
- Water-in-Oil-in-Water (W/O/W) — prepared by incorporating a W/O emulsion into water using hydrophilic surfactants.
- Oil-in-Water-in-Oil (O/W/O) — prepared by incorporating an O/W emulsion into oil using lipophilic surfactants.
Application:
Used for Sustained release of Drug– the drug that is incorporated in the innermost phase must cross to face boundaries before getting absorbed.
Emulsifying a w/o emulsion using water-soluble surfactants (which stabilize an oily dispersed phase) can produce w/o/w emulsions with an external aqueous phase, which generally has a lower viscosity than the primary w/o emulsion. Oil-in-water-in-oil (o/w/o) type multiple emulsions on the other hand consist of very small droplets of oil dispersed in the water globules of a water-in-oil emulsion.
💊 Summary Table
| Basis of Classification | Type of Emulsion | Key Features | Examples / Notes |
|---|---|---|---|
| Internal & External Phase | O/W, W/O | Based on continuous and dispersed phase | O/W – internal use; W/O – external use |
| Globule Size | Micro, Fine | Depends on droplet size | Micro (0.01 µm, transparent), Fine (0.25–25 µm, milky) |
| Structural Complexity | Multiple Emulsions | Emulsions within emulsions | W/O/W, O/W/O – for sustained release |
🧴 Conclusion
Understanding the types of pharmaceutical emulsions is essential for designing stable and effective formulations. Factors such as the nature of the phase, droplet size, and emulsifier selection play crucial roles in determining the stability, application, and drug release behavior of emulsions.
🧾 MCQs on Types of Pharmaceutical Emulsion
1. In an oil-in-water (O/W) emulsion, the continuous phase is:
A) Oil
B) Water
C) Alcohol
D) Air
✅ Answer: B) Water
2. In a water-in-oil (W/O) emulsion, the continuous phase is:
A) Water
B) Alcohol
C) Oil
D) None
✅ Answer: C) Oil
3. The typical droplet size of a microemulsion is around:
A) 0.1 mm
B) 0.01 µm
C) 10 µm
D) 100 µm
✅ Answer: B) 0.01 µm
4. Which of the following is a hydrophilic emulsifier?
A) Sorbitan esters (Spans)
B) Cholesterol
C) Sodium lauryl sulfate
D) Wool fat
✅ Answer: C) Sodium lauryl sulfate
5. Which of the following emulsions appears transparent?
A) Fine emulsion
B) Coarse emulsion
C) Microemulsion
D) Multiple emulsion
✅ Answer: C) Microemulsion
6. Multiple emulsions can be described as:
A) Simple biphasic systems
B) Emulsions within emulsions
C) Solutions
D) Suspensions
✅ Answer: B) Emulsions within emulsions
7. The particle size of a fine emulsion typically ranges between:
A) 0.01 to 0.1 µm
B) 0.25 to 25 µm
C) 1 to 100 µm
D) 100 to 500 µm
✅ Answer: B) 0.25 to 25 µm
8. Which of the following emulsions is mainly used for external application?
A) O/W emulsion
B) W/O emulsion
C) Microemulsion
D) Fine emulsion
✅ Answer: B) W/O emulsion
9. W/O/W emulsions are prepared by:
A) Mixing oil with microemulsion
B) Incorporating W/O emulsion into water
C) Mixing water and oil directly
D) Using alcohol as solvent
✅ Answer: B) Incorporating W/O emulsion into water
10. O/W/O emulsions are prepared by:
A) Incorporating O/W emulsion into oil
B) Adding surfactant to water
C) Mixing oil and water directly
D) None of the above
✅ Answer: A) Incorporating O/W emulsion into oil
11. Which of the following is a lipophilic emulsifier?
A) Sodium oleate
B) Triethanolamine stearate
C) Sorbitan ester (Span)
D) Sodium lauryl sulfate
✅ Answer: C) Sorbitan ester (Span)
12. Which emulsion type is best suited for sustained release formulations?
A) O/W emulsion
B) W/O emulsion
C) Multiple emulsion
D) Microemulsion
✅ Answer: C) Multiple emulsion
13. The milky appearance of a fine emulsion is due to:
A) Presence of oil
B) Large droplet size causing light scattering
C) Presence of surfactant
D) High viscosity
✅ Answer: B) Large droplet size causing light scattering
14. The emulsifier used in O/W emulsions should be:
A) Lipophilic
B) Hydrophilic
C) Both
D) None
✅ Answer: B) Hydrophilic
15. The term “emulsion within emulsion” refers to:
A) Simple emulsion
B) Microemulsion
C) Multiple emulsion
D) Fine emulsion
✅ Answer: C) Multiple emulsion
🧠 FAQs on Types of Pharmaceutical Emulsion
1. What are pharmaceutical emulsions?
Pharmaceutical emulsions are biphasic systems in which one immiscible liquid is dispersed as small globules within another liquid using an emulsifying agent.
2. What are the main types of pharmaceutical emulsions?
The main types are oil-in-water (O/W), water-in-oil (W/O), microemulsions, fine emulsions, and multiple emulsions.
3. What is an oil-in-water (O/W) emulsion?
In an O/W emulsion, oil is the dispersed phase and water is the continuous phase. It is commonly used for oral and injectable formulations.
4. What is a water-in-oil (W/O) emulsion?
In a W/O emulsion, water is the dispersed phase and oil is the continuous phase. These are mainly used for topical or external preparations.
5. What is the difference between O/W and W/O emulsions?
O/W emulsions have water as the continuous phase, while W/O emulsions have oil as the continuous phase. Their applications differ based on the external phase.
6. What are microemulsions?
Microemulsions are thermodynamically stable systems with very small droplet size (about 0.01 µm) and a transparent appearance.
7. What are fine emulsions?
Fine emulsions have droplet sizes ranging from 0.25 to 25 µm and appear milky due to their light-scattering globules.
8. What are multiple emulsions?
Multiple emulsions are complex systems that contain another emulsion within their dispersed phase — examples include W/O/W and O/W/O types.
9. What is the pharmaceutical application of multiple emulsions?
They are mainly used for sustained or controlled drug release, as the drug must cross multiple phase boundaries before absorption.
10. What are examples of emulsifying agents used in pharmacy?
Hydrophilic emulsifiers: sodium lauryl sulfate, triethanolamine stearate.
Lipophilic emulsifiers: sorbitan esters (Spans), cholesterol, wool fat, calcium palmitate.
